16
2 Safety
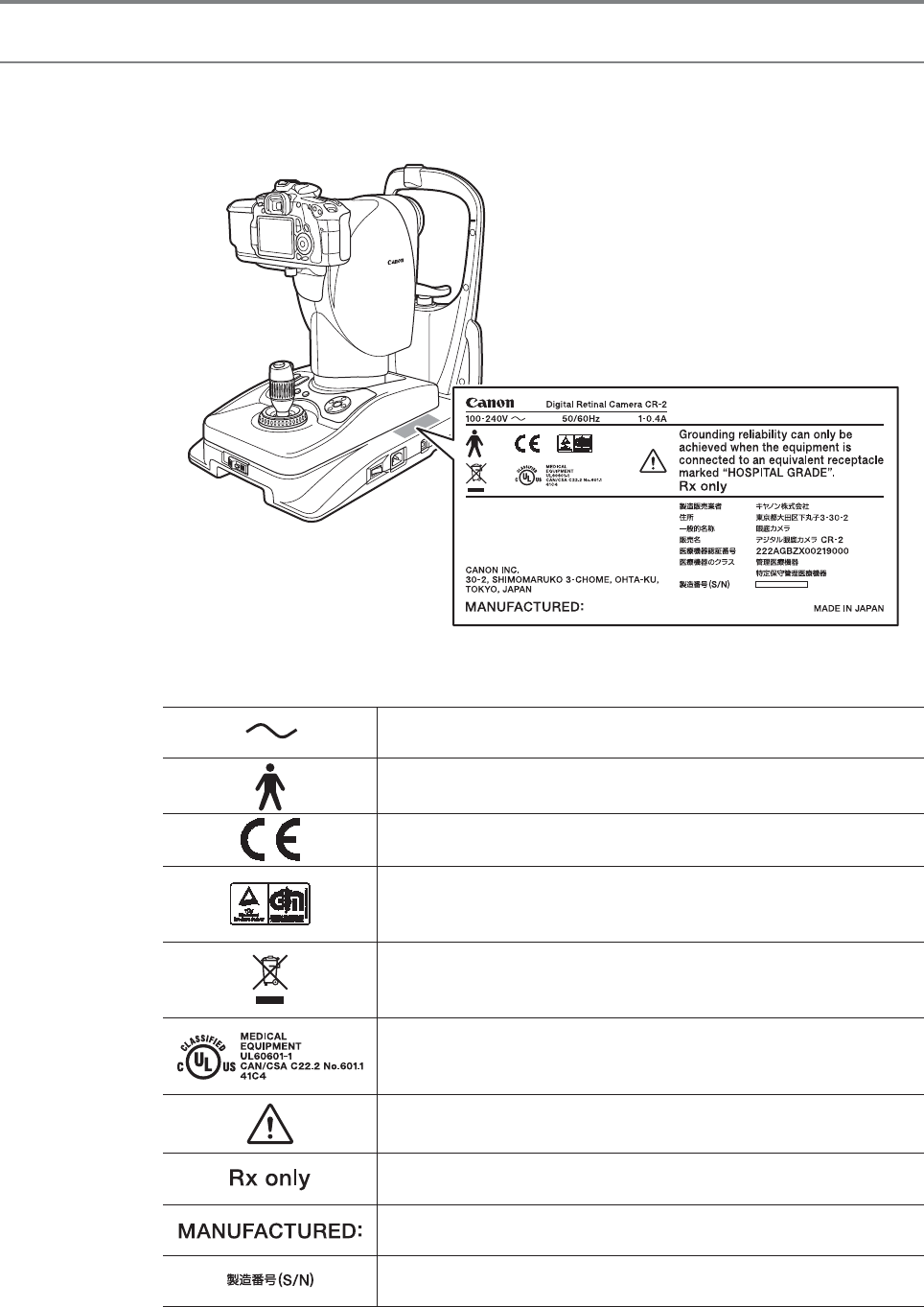
Rating Label Display
The position and contents of the label attached on the CR-2 is shown below.
Follow the information on the label to use the CR-2 appropriately.
The following table describes the marks and indications on the rating label.
Alternating current
Type B
Class I device indicating manufacturer’s declaration of conformance
with the Annex VII of the Medical Device Directive, 93/42/EEC
Certification mark that shows the product has passed the tests by
TUV Rheinland of conformity with the medical device regulations in the
European Union.
Product that WEEE directive, Directive on Waste Electrical and
Electronic Equipment, 2002/96/EC, requires separate collection. The
directive is effective in the European Union only.
Certification mark that indicates the product complies with UL 60601-
1 and CAN/CSA 22.2 No. 601.1, that specifies protection against fire,
electric shock, and mechanical hazards
Caution: Check the documentation provided.
Caution: Federal law (USA) restricts this device to sale by or on the
order of a physician.
Year and month of manufacture Example: October 2011
Serial number in six digits Example: 123456


















