20020601
English
Activity: SetupActivity: Setup
2-4-1
í Equipment
Syringe (with scale markings) Plastic Container Rubber Tube
Rubber Gasket Clip Mixing Stick
Warm Water, Cold Water, Ice
Temperature Measurement Setup (EA-200, graphic scientific calculator,
data communication cable, temperature probe)
í Assembling the Equipment
u Cut a hole into the side of the plastic container, and affix the rubber gasket around the
hole on the outside of the container.
u Slip the syringe with the rubber tube on its tip into the hole, and pack it with rubber to
make it watertight.
u Affix the clip to the rubber tube to seal the air inside the syringe.
í Setting Up
u Fill the plastic container with warm water and wait until the air in the syringe stabilizes.
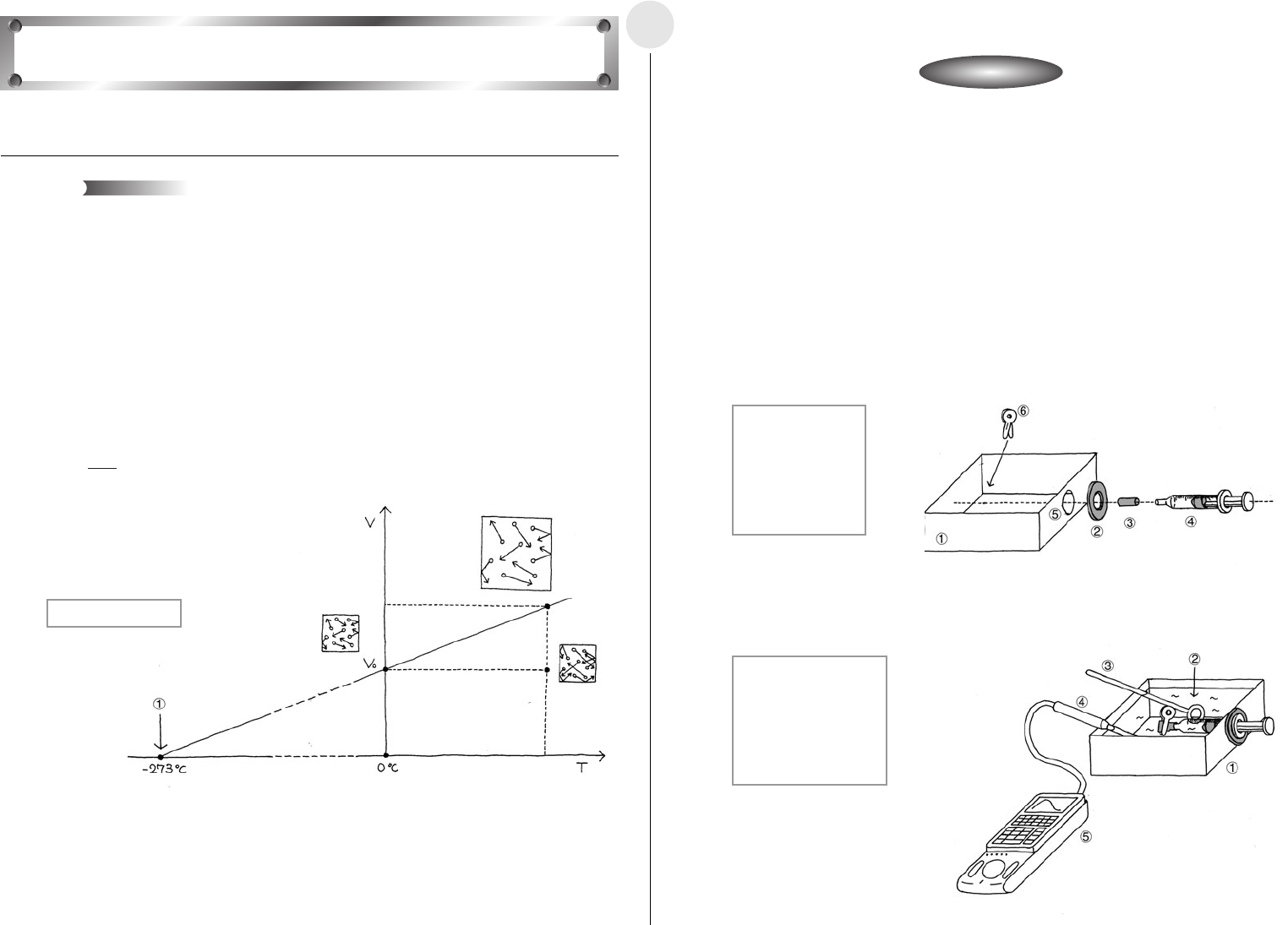
This activity is designed to confirm Charles’ law through an actual experiment.
Increasing the temperature of a gas causes the molecules that make up the gas move
faster. The pressure within the container that holds the gas is determined by the number of
collisions between the molecules and the walls of the container, and by the velocity of the
molecules when they collide with the walls. If pressure remains constant and temperature
increases, the gas expands, which reduces the number of molecular impacts with the
container walls and negates the increase in molecular velocity.
Charles’ Law states that the thermal expansion of rarified gas of constant pressure is
proportional to the increase in temperature, and is represented by the expression shown
below. If the temperature when the volume of gas reaches zero is defined as absolute zero,
absolute zero is –273°C.
V(m
3
):Gas Volume
V
0
(m
3
):Gas Volume at 0°C
T(°C) : Gas Temperature
Charles’ Law
Theory
V = (T + 273)
V
0
273
1 Plastic Container
2 Rubber Gasket
3 Rubber Tube
4 Syringe
5 Hole
6 Clip
1 Plastic Container
2 Warm Water: 60°C
3 Mixing Stick
4 Temperature Probe
(CH1)
5 EA-200
1 Absolute Zero


















