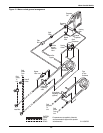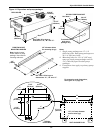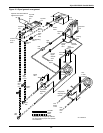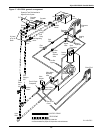
Glycol/GLYCOOL-Cooled Models
30
5.5.2 Glycol Solutions
When considering the use of any glycol products in a particular application, you should review the lat-
est Material Safety Data Sheets and ensure that the use you intend can be accomplished safely. For
Material Safety Data Sheets and other product safety information, contact the supplier nearest you.
Before handling any other products mentioned in the text, you should obtain available product safety
information and take necessary steps to ensure safety of use.
NOTICE
When mishandled, glycol products pose a threat to the environment. Before using any glycol
products, review the latest Material Safety Data Sheets and ensure that you can use the
product safely.
Glycol manufacturers request that the customer read, understand and comply with the
information on the product packaging and in the current Material Safety Data Sheets. Make
this information available to anyone responsible for operation, maintenance and repair of the
drycooler and related equipment.
No chemical should be used as or in a food, drug, medical device, or cosmetic, or in a product or pro-
cess in which it may contact a food, drug, medical device, or cosmetic until the user has determined
the suitability and legality of the use. Since government regulations and use conditions are subject to
change, it is the user's responsibility to determine that this information is appropriate and suitable
under current, applicable laws and regulations.
NOTICE
Automotive antifreeze is unacceptable and must NOT be used.
Typical inhibited formula ethylene glycol and propylene glycol manufacturers and suppliers are
Union Carbide (Ucartherm) or Dow Chemical (Dowtherm SR-1, Dowfrost). These glycols are supplied
with corrosion inhibitors and do not contain a silicone anti-leak formula. Commercial ethylene glycol,
when pure, is generally less corrosive to the common metals of construction than water itself. Aque-
ous solutions of these glycols, however, assume the corrosivity of the water from which they are pre-
pared and may become increasingly corrosive with use if not properly inhibited.
There are two basic types of additives: corrosion inhibitors and environmental stabilizers. The corro-
sion inhibitors function by forming a surface barrier that protects the metals from attack. Environ-
mental stabilizers, while not corrosion inhibitors in the strictest sense of the word, decrease corrosion
by stabilizing or favorably altering the overall environment. An alkaline buffer such as borax is a sim-
ple example of an environmental stabilizer since its prime purpose is to maintain an alkaline condi-
tion (pH above 7).
The percentage of glycol to water must be determined by using the lowest design outdoor temperature
in which the system is operating. Table 14 indicates the solution freeze point at several concentra-
tion levels of ethylene glycol. Propylene glycol concentrations should be 1% higher than the ethylene
glycol table values to find the freeze point. For example, 41% propylene glycol freezes at -10°F (-23°C).
NOTICE
The quality of water used for dilution must be considered because water may contain corrosive
elements that reduce the effectiveness of the inhibited formulation. Water classified as soft
(low in chloride and sulfate ion content less than 100 parts per million each) should be used.
NOTE
Glycol solutions should be considered for protection of the coil. When it is not used, damage can
occur from either freezing or corrosion from water.
Table 14 Ethylene glycol concentrations
% Glycol by Volume 0 * 10 20 30 40 50
Freezing Point °F (°C) 32 (0) 25 (-3.9) 16 (-8.9) 5 (-15.0) -10 (-23.3) -32 (-35.5)
Apparent Specific Gravity
@ 50°F (10°C)
1 1.014 1.028 1.042 1.057 1.071
* A minimal amount of glycol should be considered for inhibitive coil protection.