20020601
English
Activity: SetupActivity: Setup
2-11-1
í Equipment
Stand Heater Reflux Condenser Desiccant
Auto Stirrer Beaker Round Bottom Flask (2) Mixing Stick
Ice Water Benzene Naphthalene
Temperature Measurement Setup (EA-200, graphic scientific calculator, data
communication cable, temperature probe)
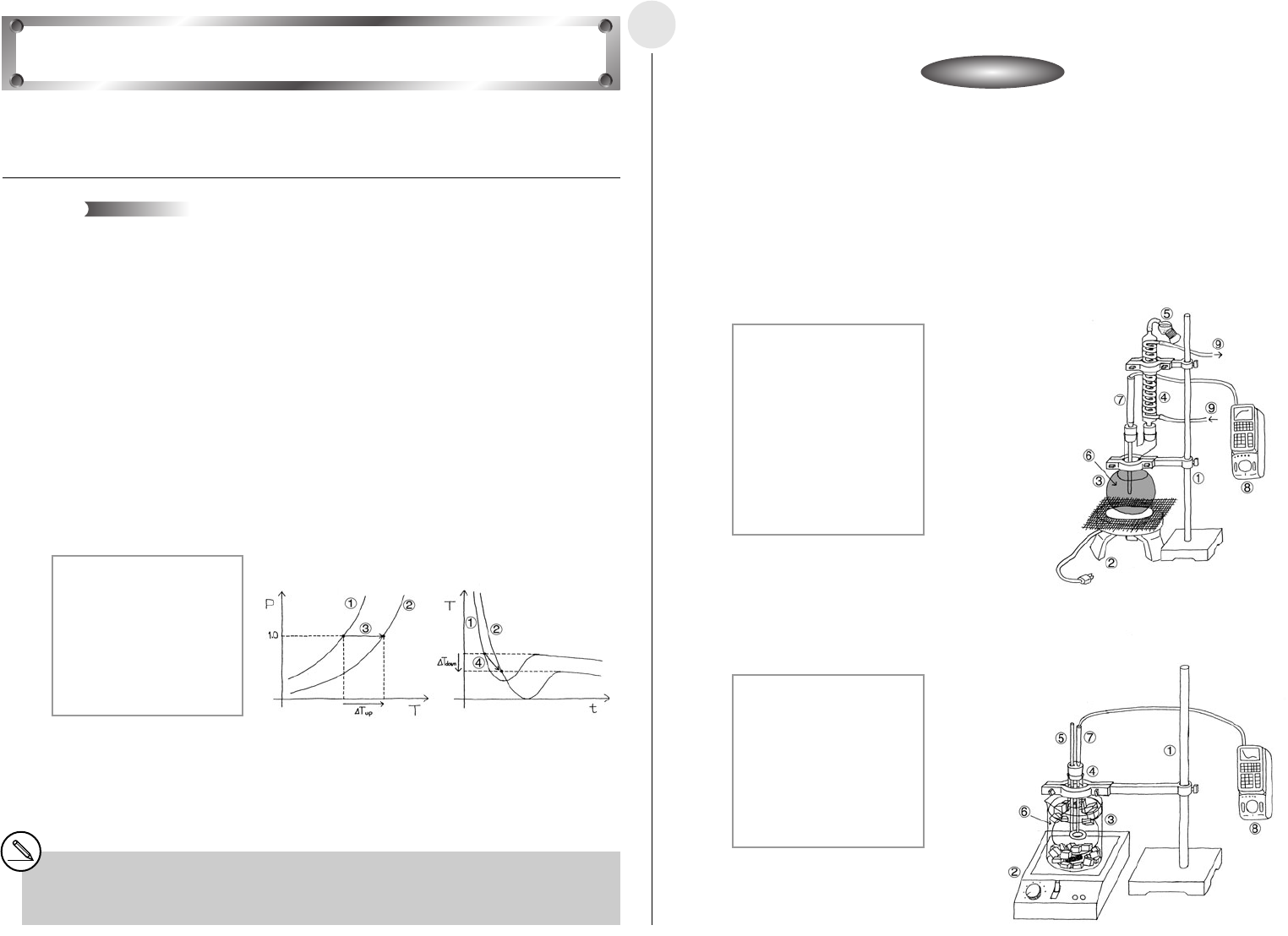
í Setting Up the Boiling Point Elevation Equipment
u Pour the benzene solution into the flask, and secure it in place as shown in the illustration.
This activity investigates boiling point elevation and freezing point depression of a dilute
solution.
Dissolving a small amount of a substance (solute) in a theoretically pure liquid (solvent) to
create a dilute solution causes the boiling point of the dilute solution to become greater than
and the freezing point to become less than that of the solvent. This is because the
proportion of solvent molecules is reduced by the amount of solute molecules mixed in,
which lowers the vapor pressure of the solvent and elevates the boiling point. At the same
time, it also reduces the proportion of solvent molecules that congeal, which suppresses the
freezing point. These changes are determined by the amount of solute molecules, and the
type of solute does not matter, as long as it is non-volatile. Consequently, both boiling point
elevation and freezing point depression are proportional to the solute molality, as shown in
the expressions below.
ͬ
T
1
(°C) : Boiling Point Elevation of Solution
ͬ
T
2
(°C) : Freezing Point Depression of Solution
K
1
(°C kg/mol) : Molal Boiling Point Elevation Constant
K
2
(°C kg/mol) : Molal Freezing Point Depression
Constant
m(mol/kg) : Molality
Here, the proportion coefficient is determined by the solvent type. It is a constant that is not
affected by the solute type. For example, the molal boiling point elevation constant for benzene
is 2.53°C kg/mol, and the boiling point is 80.1°C. The molal freezing point depression constant
is 5.12°C kg/mol, and the freezing point is 5.53°C.
Dilute Solution Properties
Theory
When the solvent is pure water, the molal boiling point elevation constant is 0.515°C kg/mol,
and the boiling point is 100°C. The molal freezing point depression constant is 1.853°C kg/mol,
and the freezing point is 0°C.
í Setting Up the Freezing Point Depression Equipment
u Pour the benzene solution into the flask, and secure it in place as shown in the illustration.
1 Stand
2 Heater
3 Round Bottom Flask
4 Reflux Condenser
5 Desiccant
6 Naphthalene-Benzene
Solution
7 Temperature Probe (CH1)
8 EA-200
9 Water Flow Direction
1 Stand
2 Auto Stirrer
3 Beaker
4 Round Bottom Flask
5 Mixing Stick
6 Ice Water
7 Temperature Probe (CH1)
8 EA-200
1 Solvent
2 Solution
3 Boiling Point Elevation
4 Freezing Point Depression
T(°C) : Temperature
P(atm) : Vapor Pressure
t(s) : Time
ͬT
1
= K
1
m
ͬT
2
= K
2
m


















