20020601
English
Activity: Operating the EquipmentActivity: Operating the Equipment
MeasurementMeasurement
2-12-2
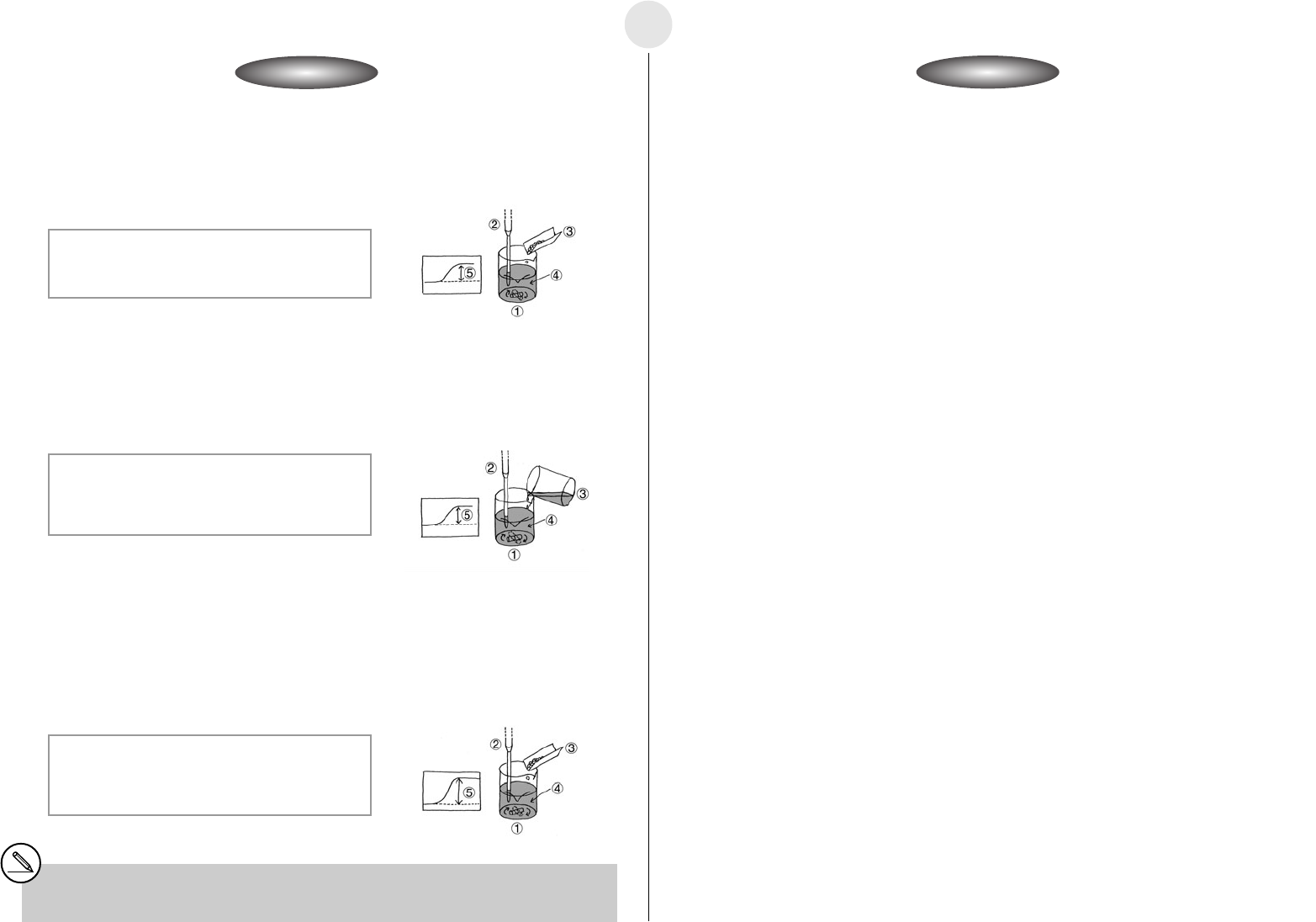
í Reaction Path 1 Measurement
u Turn on the auto stirrer and start a measurement operation with the calculator.
u Little-by-little, add sodium hydroxide (
s) to the distilled water, and observe how the
temperature changes.
u After all the sodium hydroxide (
s) is dissolved and the temperature rise stabilizes,
determine the heat of dissolution.
u Turn on the auto stirrer and start a measurement operation with the calculator.
u Little-by-little, add the hydrochloric acid (
aq) to the sodium hydroxide (aq), and observe
how the temperature changes.
u After all the hydrochloric acid (
aq) is added and the temperature rise stabilizes, determine
the heat of neutralization.
u Calculate the sum of heat by adding the heat of neutralization to the heat of dissolution.
í Reaction Path 2 Measurement
u Turn on the auto stirrer and start a measurement operation with the calculator.
u Little-by-little, add sodium hydroxide (
s) to the hydrochloric acid (aq), and observe how the
temperature changes.
u After all the sodium hydroxide (
s) is dissolved and the temperature rise stabilizes,
determine the heat of neutralization.
u Calculate the heat, and compare it with the total heat you calculated for Reaction Path 1.
55555555555555555555555
5555555555555555555555
55555
Other Things To Do
55555
í Calculator Operation
u Use the Temperature Measurement Setup to measure the temperature and then display it.
u Find the applicable program in the Program Library (P.2-16-3), input it into your calculator,
and then run it.
1 Beaker
2 Temperature Probe
3 Sodium Hydroxide (s)
u Determine whether this activity verifies Hess’s Law. If it does not, consider the
reason why.
u Compare the theoretical chemical reaction expression and the measurement
results. If they do not match, consider the reason why.
u Find out if Hess’s Law holds true for other chemical reactions.
1 Beaker
2 Temperature Probe
3 Hydrochloric Acid
(aq)
1 Beaker
2 Temperature Probe
3 Sodium Hydroxide
(s)
4 Distilled Water
5 Temperature rise due
to heat of dissolution
4 Sodium Hydroxide (aq)
5 Temperature rise due
to heat of neutralization
4 Hydrochloric Acid (aq)
5 Temperature rise due
to heat of neutralization
When converting the temperature rise to joule heat, be sure to take the difference in the volume of
water into consideration. The specific heat of distilled water is 4.2J/g°C.


















