A-22
FDA Regulations (For 110-120 V Model Only)
U.S. Food and Drug Administration (FDA) has implemented regulations for laser products
manufactured on and after August 2, 1976. Compliance is mandatory for products marketed in
the United States. One of the following labels on the back of the printer indicates compliance
with the FDA regulations and must be attached to laser products marketed in the United States.
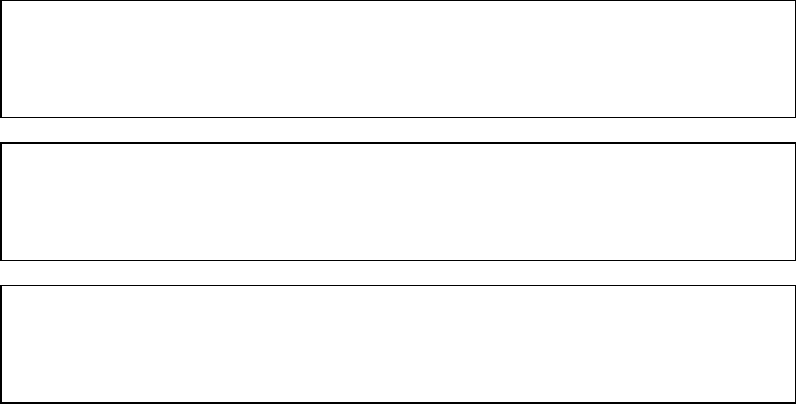
MANUFACTURED:
Brother Corporation (Asia) Ltd. Brother Buji Nan Ling Factory
Gold Garden Ind., Nan Ling Village, Buji, Rong Gang, Shenzhen, CHINA
This product complies with FDA radiation performance standards, 21 CFR Subchapter J
MANUFACTURED:
BROTHER INDUSTRIES (USA) INC.
2950 Brother Blvd., Bartlett, TN 38133, U.S.A.
This product complies with FDA radiation performance standards, 21 CFR Subchapter J
MANUFACTURED:
BROTHER INDUSTRIES LTD.
15-1 Naeshiro-cho Mizuho-ku Nagoya, 467-8561 Japan
This product complies with FDA radiation performance standards, 21 CFR Subchapter J
Caution
Use of controls, adjustments or performance of procedures other than those specified in this
manual may result in hazardous radiation exposure.
Declaration of Conformity (For Europe)
We, Brother Industries, Ltd.,
15-1, Naeshiro-cho, Mizuho-ku, Nagoya 467-8561, Japan
declare that this product is in conformity with the following normative documents.
Safety: EN 60950, EN 60825
EMC: EN 55022 Class B, EN 55024
EN 61000-3-2, EN 61000-3-3
following the provisions of the Low Voltage Directive 73/23/EEC and the Electromagnetic
Compatibility Directive 89/336/EEC (as amended by 91/263/EEC and 92/31/EEC).
Issued by:
Brother Industries, Ltd.
Printer Products Division


















