MEASURING PRINCIPLE
2 - 9
ETC00303(1) BINOS E e (2.0) 11/00
Rosemount Analytical
2.3.2 Electrochemical Measurement
The determination of O
2
concentrations is based on the principle of a galvanic cell.
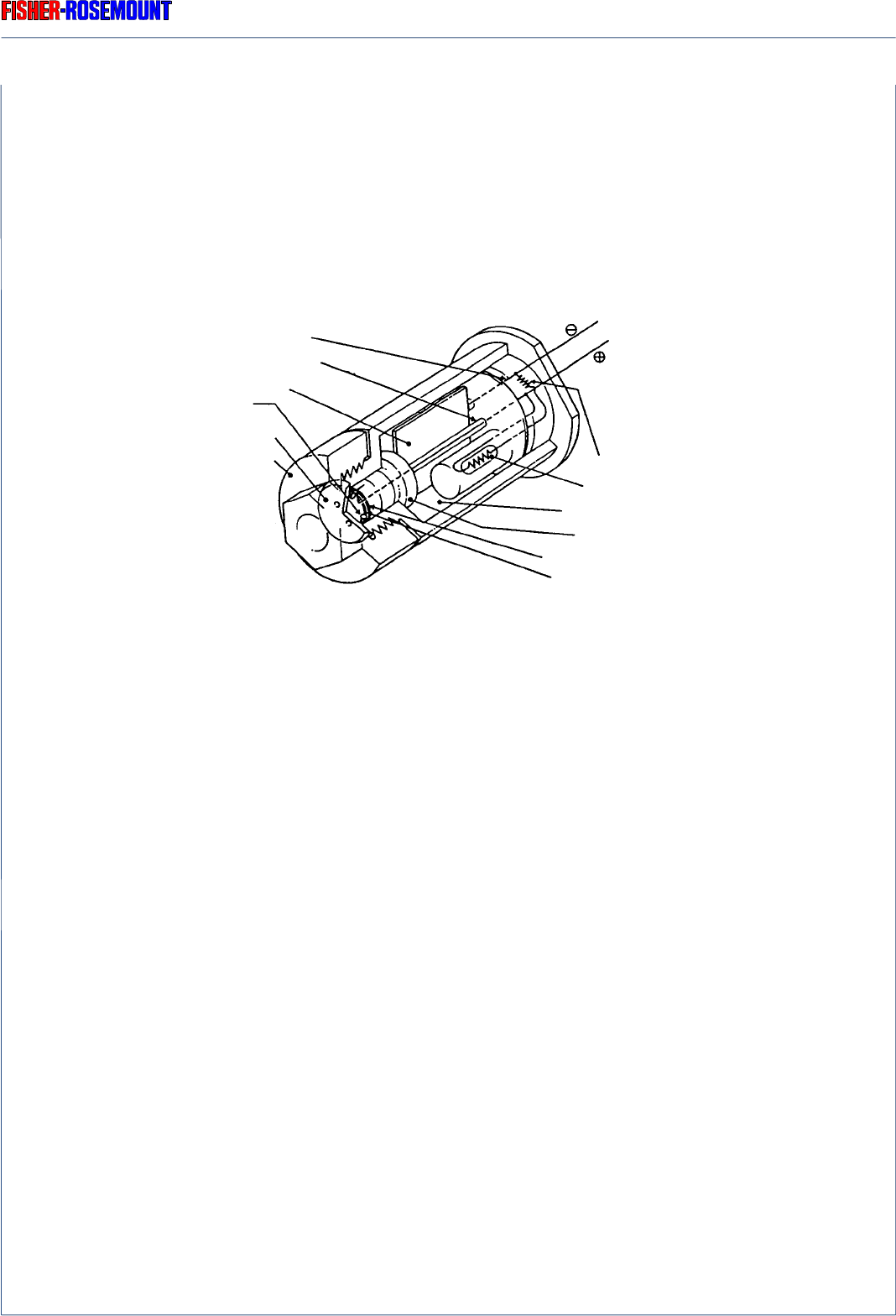
The principle structure of the oxygen sensor is shown in Fig. 2-5.
Lead wire (Anode)
Lead wire (Cathode)
O ring (8)
Plastic disc (9)
Plastic top (10)
Resistor (6)
Thermistor (5)
Acid electrolyte (3)
(Black)
(Red)
Fig. 2-5: Structure of electrochemical Oxygen Sensor
The oxygen sensor incorporates a lead/gold oxygen cell with a lead anode (1) and a gold
cathode (2), using a specific acid electrolyte. To avoid moisture losses at the gold electrode a
sponge sheet is inserted on the purged side.
Oxygen molecules diffuse through a non-porous Teflon membrane (4) into the electrochemical
cell and are reduced at the gold-cathode. Water results from this reaction.
On the anode lead oxide is formed which is transfered into the electrolyte. The lead anode is
regenerated continuously and the electrode potential therefore remains unchanged for a long
time.
The rate of diffusion and so the response time (t
90
) of the sensor is depending on the thickness
of the Teflon membrane.
OXYGEN MEASUREMENT
Teflon membrane (4)
Cathode
(2)
(Gold film)
Sponge disc (7)
Anode
(1)
(Lead)


















