Appendix B Overview of Isotopes
B-2 Applied Biosystems
B
B.1 Isotopes
Overview Many elements in their natural state exist as one of several
isotopes. An isotope is one of two or more atoms with the
same atomic number but a different mass. The most abundant
isotope of carbon is
12
C, but natural carbon also contains
13
C
and
14
C.
Because a mass spectrometer measures mass-to-charge
ratios, isotopes appear in the mass spectrum. Isotopes of low
abundance, such as
14
C, do not affect the appearance of a
mass spectrum. However, isotopes that occur in greater
abundance, such as
13
C, which occurs in a natural abundance
of approximately 1.1 percent
1
in carbon, do affect the
appearance of a mass spectrum.
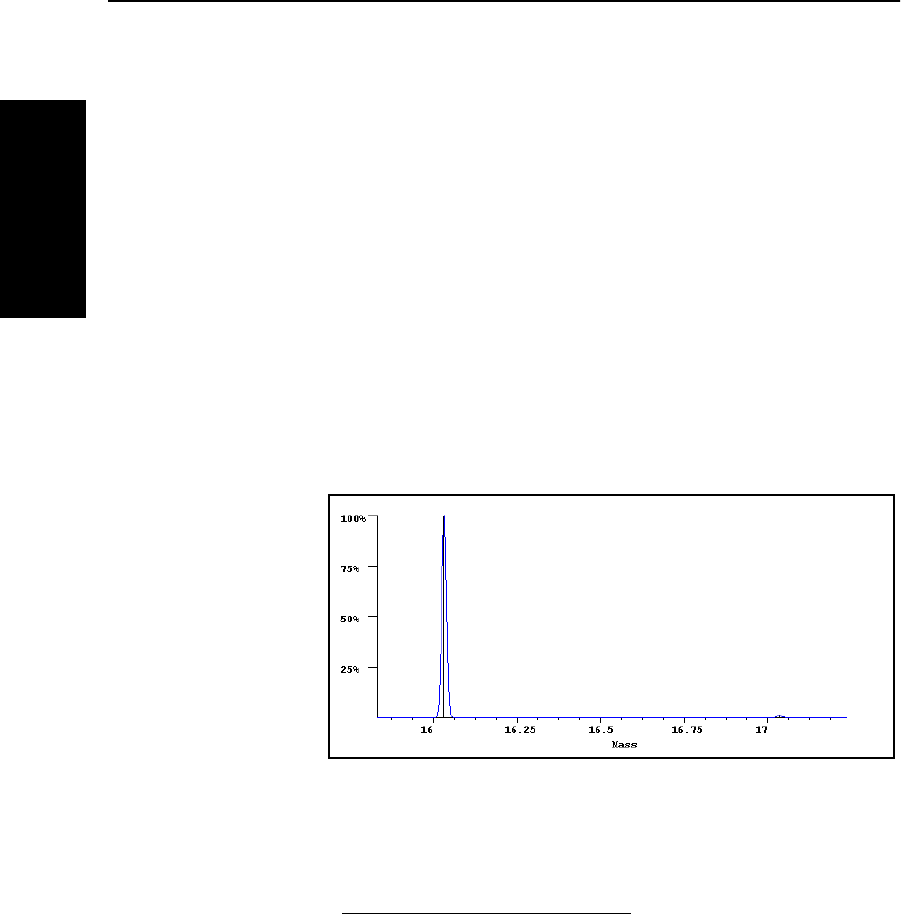
The mass spectrum of methane (Figure B-1) illustrates the
impact of an isotope on the appearance of a mass spectrum.
Methane includes a peak representing the molecular ion at
16 Da (
12
CH
4
) and a peak representing the isotope at 17 Da
(
13
CH
4
). The relative abundance of the ions is about 99:1.
Figure B-1 Mass Spectrum of Methane
1. Meth. Enzymol., McCloskey, J.A, ed., 1990, 193, 869.
12
C
13
C


















